Safety Moment #3: Flammable Range and Flash Point

The material shown here has been extracted from the ebook 52 Process Safety Moments and in the book Plant Design and Operations.
Contents
- Introduction
- Flammable Range
- Flash Point
- Flammability and Ignition Limits
- Autoignition Temperature
- Further Information
Introduction
For many process and energy facilities, fires and explosions represent the greatest potential for a catastrophic event. Hence it is vital that those responsible for the design and operation of these facilities have a proper understanding of the terms ‘Flammable Limit’ and ‘Flash Point’. Yet, all too frequently, these terms are used somewhat loosely and inaccurately.
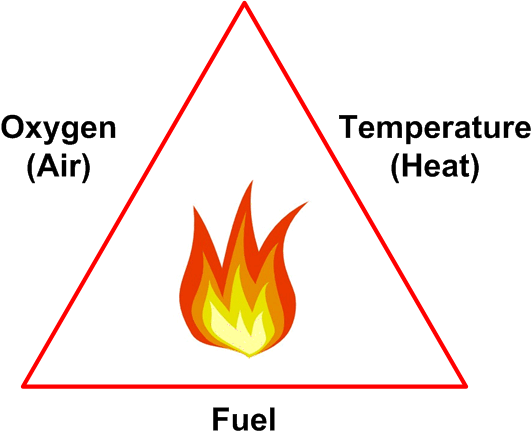
Fire triangles, such as the one shown, are often used to explain the requirements for combustion to occur. They show that there has to be a source of fuel — usually a hydrocarbon such as gasoline or natural gas. Then there has to be sufficient oxygen to sustain combustion. (The word ‘Air’ is often used in fire triangles since most fires take their oxygen from the atmosphere.) The fuel (in vapor form) and the air/oxygen mix has to be within a flammable range. Finally, the temperature of the fuel/air mix has to be above a minimum threshold value — if it is not the mixture will not burn.
Although triangles such the one shown are useful for illustrative purposes, they do have two limitations. First, the word ‘Heat’, rather than ‘Temperature’ is frequently used. This usage is misleading — it is a it is a high enough temperature that is required. A second concern to do with fire triangles is that they do not take into account the fact that the fuel and flammable material have to be within the flammable range, otherwise they will not burn.
For these reasons, more than a simple fire triangle is needed for those who are charged with designing and operating process and energy facilities.
. . . . .
You are welcome to use this Safety Moment in your workplace. But there are restrictions — please read Use of Safety Moments.
Copyright © Ian Sutton. 2018. All Rights Reserved.
